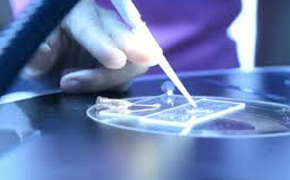
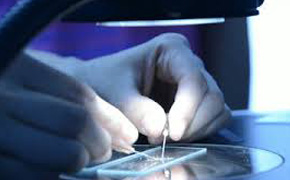
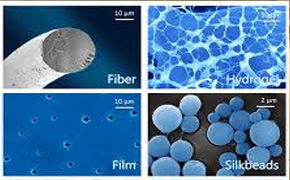
Suitable for ; Medical devices – Pharmaceuticals – Micro Electronics – Semiconductors – Pilot plants or Clinical - Biotechnology – R & D – Productions.
Phuket Island #Work with Views#
and lower cost than your country**

| Property type : | Factory |
| Land area : | 17 Rai or 6.7 acres or 26,954 sq.m. |
| Office area : | 350 sq.m. |
| Clean room area : | 650 sq.m. or 6,890 sq.ft. |
| Validated Cleanroom : | IQ, OQ, PQ and Microbiology monitoring. |
| Cleanroom class : | ISO class 8 (100000) and ISO class 7 (10000) option. |
| Canteen area : | 360 sq.m. |
| Full licensing : | Thailand FDA - Manufacturin & Medical Devices Import license ISO 13485 : 2003Quality Systems Medical Devices, ISO 9001 : 2008 Quality management systems (requirements) |
| Location : | 15 minutes to Airport, 30 minutes to deep sea port / cargo. |
| History : |  |
| Support with : | - Full management facilities. - Local material (options). - Full logistics – deep sea port, air freight and truck. |
| Human resources | 8 years experiences staff in medical devices manufacturing.
Experiences in working under microscope.
Experiences in assembling micron product size.
Experiences in management quality system and FDA regulatory.
Staff recruitment within 2 weeks, training period 4 weeks.
Production operator salary THB15,000-THB20,000/month, 5 working days/week
Local management team, 25years experience in multinational manufacturing of both electronics and medical devices. |